Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric chargewhereas the electrons have a negative electric charge. The neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively. These atoms are called ions.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus shatters and leaves behind different elements. This is a kind of nuclear decay. All electrons, nucleons, and nuclei alike are subatomic particles. The behavior of electrons in atoms is closer to a wave than a particle.
The number of protons in the nucleus, called the atomic number, defines to which chemical element the atom belongs. For example, each copper atom contains 29 protons. The number of neutrons defines the isotope of the element. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature. Chemistry is the discipline that studies these changes.The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word atomos, meaning "uncuttable", was coined by the ancient Greek philosophersLeucippus and his pupil Democritus (c. 460 – c. 370 BC).[1][2][3][4] Democritus taught that atoms were infinite in number, uncreated, and eternal, and that the qualities of an object result from the kind of atoms that compose it.[2][3][4] Democritus's atomism was refined and elaborated by the later philosopher Epicurus (341–270 BC).[3][4] During the Early Middle Ages, atomism was mostly forgotten in western Europe, but survived among some groups of Islamic philosophers.[3] During the twelfth century, atomism became known again in western Europe through references to it in the newly-rediscovered writings of Aristotle.[3]
In the fourteenth century, the rediscovery of major works describing atomist teachings, including Lucretius's De rerum natura and Diogenes Laërtius's Lives and Opinions of Eminent Philosophers, led to increased scholarly attention on the subject.[3]Nonetheless, because atomism was associated with the philosophy of Epicureanism, which contradicted orthodox Christian teachings, belief in atoms was not considered acceptable.[3] The French Catholic priest Pierre Gassendi (1592–1655) revived Epicurean atomism with modifications, arguing that atoms were created by God and, though extremely numerous, are not infinite.[3][4] Gassendi's modified theory of atoms was popularized in France by the physician François Bernier (1620–1688) and in England by the natural philosopher Walter Charleton (1619–1707).[3] The chemist Robert Boyle (1627–1691) and the physicist Isaac Newton (1642–1727) both defended atomism and, by the end of the seventeenth century, it had become accepted by portions of the scientific community
First evidence-based theory
Various atoms and molecules as depicted in John Dalton's A New System of Chemical Philosophy (1808).
In the early 1800s, John Dalton used the concept of atoms to explain why elementsalways react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in multiples of discrete units — in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[5]
Dalton also believed atomic theory could explain why water absorbs different gases in different proportions. For example, he found that water absorbs carbon dioxide far better than it absorbs nitrogen.[6] Dalton hypothesized this was due to the differences between the masses and configurations of the gases' respective particles, and carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
In the early 1800s, John Dalton used the concept of atoms to explain why elementsalways react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in multiples of discrete units — in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[5]
Brownian motion
In 1827, botanist Robert Brown used a microscope to look at dust grains floating in water and discovered that they moved about erratically, a phenomenon that became known as "Brownian motion". This was thought to be caused by water molecules knocking the grains about. In 1905, Albert Einstein proved the reality of these molecules and their motions by producing the first statistical physics analysis of Brownian motion.[7][8][9]French physicist Jean Perrin used Einstein's work to experimentally determine the mass and dimensions of atoms, thereby conclusively verifying Dalton's atomic theory.[10]
Discovery of the electron
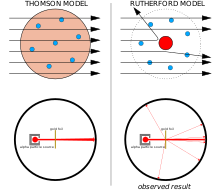
The Geiger–Marsden experiment
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
The physicist J.J. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen. Therefore, they were not atoms, but a new particle, the first subatomic particle to be discovered, which he originally called "corpuscle" but was later named electron, after particles postulated by George Johnstone Stoney in 1874. He also showed they were identical to particles given off by photoelectricand radioactive materials.[11] It was quickly recognized that they are the particles that carry electric currents in metal wires, and carry the negative electric charge within atoms. Thomson was given the 1906 Nobel Prize in Physics for this work. Thus he overturned the belief that atoms are the indivisible, ultimate particles of matter.[12]Thomson also incorrectly postulated that the low mass, negatively charged electrons were distributed throughout the atom in a uniform sea of positive charge. This became known as the plum pudding model.

The Geiger–Marsden experiment
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.


No comments:
Post a Comment