Decorative paint work is one of the modern and current/trading paints work it has many advantages over normal paint work which help in having long lasting solution to some problems that faces paint works in buildings.
painting works has been facing alot of problem in the previous years due to poor quality materials used in building construction works such as rod, cements, and also poor missing of cements and poor mixture of cements with stones and sand.
we will be discussing some of the problem painting is facing for over 10 years.
these are the out look of Decoretive painted walls.
The decorative painted wall have the ability to last over 20 years. It has 100% washable ability just like motor body.
Decorative painting works comes on different design and colors and surfaces which is very attractive surface to the test of any individual.
the material is also foreign and imported....
PROFESSIONAL SERVICES
*Delivering the best on building Contract, such as house, companies, schools, Churches, market, house estate, complex, plaza, etc. *Production of all kinds is high quality paints, such Emulsion silk paint, Text - Coat paints, emulsion grade 3 paints, Decorative paints, etc. *Service: painting of any style and design, installation of 3D wall paper & panels, printing of different design, etc And lot more
Friday, January 15, 2021
Tuesday, September 3, 2019
Atomic solution and structural particules
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons. Protons and neutrons are called nucleons. More than 99.94% of an atom's mass is in the nucleus. The protons have a positive electric chargewhereas the electrons have a negative electric charge. The neutrons have no electric charge. If the number of protons and electrons are equal, then the atom is electrically neutral. If an atom has more or fewer electrons than protons, then it has an overall negative or positive charge, respectively. These atoms are called ions.
The electrons of an atom are attracted to the protons in an atomic nucleus by the electromagnetic force. The protons and neutrons in the nucleus are attracted to each other by the nuclear force. This force is usually stronger than the electromagnetic force that repels the positively charged protons from one another. Under certain circumstances, the repelling electromagnetic force becomes stronger than the nuclear force. In this case, the nucleus shatters and leaves behind different elements. This is a kind of nuclear decay. All electrons, nucleons, and nuclei alike are subatomic particles. The behavior of electrons in atoms is closer to a wave than a particle.
The number of protons in the nucleus, called the atomic number, defines to which chemical element the atom belongs. For example, each copper atom contains 29 protons. The number of neutrons defines the isotope of the element. Atoms can attach to one or more other atoms by chemical bonds to form chemical compounds such as molecules or crystals. The ability of atoms to associate and dissociate is responsible for most of the physical changes observed in nature. Chemistry is the discipline that studies these changes.The idea that matter is made up of discrete units is a very old idea, appearing in many ancient cultures such as Greece and India. The word atomos, meaning "uncuttable", was coined by the ancient Greek philosophersLeucippus and his pupil Democritus (c. 460 – c. 370 BC).[1][2][3][4] Democritus taught that atoms were infinite in number, uncreated, and eternal, and that the qualities of an object result from the kind of atoms that compose it.[2][3][4] Democritus's atomism was refined and elaborated by the later philosopher Epicurus (341–270 BC).[3][4] During the Early Middle Ages, atomism was mostly forgotten in western Europe, but survived among some groups of Islamic philosophers.[3] During the twelfth century, atomism became known again in western Europe through references to it in the newly-rediscovered writings of Aristotle.[3]
In the fourteenth century, the rediscovery of major works describing atomist teachings, including Lucretius's De rerum natura and Diogenes Laërtius's Lives and Opinions of Eminent Philosophers, led to increased scholarly attention on the subject.[3]Nonetheless, because atomism was associated with the philosophy of Epicureanism, which contradicted orthodox Christian teachings, belief in atoms was not considered acceptable.[3] The French Catholic priest Pierre Gassendi (1592–1655) revived Epicurean atomism with modifications, arguing that atoms were created by God and, though extremely numerous, are not infinite.[3][4] Gassendi's modified theory of atoms was popularized in France by the physician François Bernier (1620–1688) and in England by the natural philosopher Walter Charleton (1619–1707).[3] The chemist Robert Boyle (1627–1691) and the physicist Isaac Newton (1642–1727) both defended atomism and, by the end of the seventeenth century, it had become accepted by portions of the scientific community
First evidence-based theory
Various atoms and molecules as depicted in John Dalton's A New System of Chemical Philosophy (1808).
In the early 1800s, John Dalton used the concept of atoms to explain why elementsalways react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in multiples of discrete units — in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[5]
Dalton also believed atomic theory could explain why water absorbs different gases in different proportions. For example, he found that water absorbs carbon dioxide far better than it absorbs nitrogen.[6] Dalton hypothesized this was due to the differences between the masses and configurations of the gases' respective particles, and carbon dioxide molecules (CO2) are heavier and larger than nitrogen molecules (N2).
In the early 1800s, John Dalton used the concept of atoms to explain why elementsalways react in ratios of small whole numbers (the law of multiple proportions). For instance, there are two types of tin oxide: one is 88.1% tin and 11.9% oxygen and the other is 78.7% tin and 21.3% oxygen (tin(II) oxide and tin dioxide respectively). This means that 100g of tin will combine either with 13.5g or 27g of oxygen. 13.5 and 27 form a ratio of 1:2, a ratio of small whole numbers. This common pattern in chemistry suggested to Dalton that elements react in multiples of discrete units — in other words, atoms. In the case of tin oxides, one tin atom will combine with either one or two oxygen atoms.[5]
Brownian motion
In 1827, botanist Robert Brown used a microscope to look at dust grains floating in water and discovered that they moved about erratically, a phenomenon that became known as "Brownian motion". This was thought to be caused by water molecules knocking the grains about. In 1905, Albert Einstein proved the reality of these molecules and their motions by producing the first statistical physics analysis of Brownian motion.[7][8][9]French physicist Jean Perrin used Einstein's work to experimentally determine the mass and dimensions of atoms, thereby conclusively verifying Dalton's atomic theory.[10]
Discovery of the electron
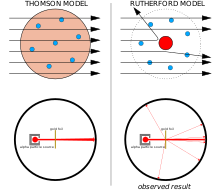
The Geiger–Marsden experiment
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Right: Observed results: a small portion of the particles were deflected by the concentrated positive charge of the nucleus.
The physicist J.J. Thomson measured the mass of cathode rays, showing they were made of particles, but were around 1800 times lighter than the lightest atom, hydrogen. Therefore, they were not atoms, but a new particle, the first subatomic particle to be discovered, which he originally called "corpuscle" but was later named electron, after particles postulated by George Johnstone Stoney in 1874. He also showed they were identical to particles given off by photoelectricand radioactive materials.[11] It was quickly recognized that they are the particles that carry electric currents in metal wires, and carry the negative electric charge within atoms. Thomson was given the 1906 Nobel Prize in Physics for this work. Thus he overturned the belief that atoms are the indivisible, ultimate particles of matter.[12]Thomson also incorrectly postulated that the low mass, negatively charged electrons were distributed throughout the atom in a uniform sea of positive charge. This became known as the plum pudding model.

The Geiger–Marsden experiment
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Left: Expected results: alpha particles passing through the plum pudding model of the atom with negligible deflection.
Discovery of the nucleus
Main article: Geiger-Marsden experiment
In 1909, Hans Geiger and Ernest Marsden, under the direction of Ernest Rutherford, bombarded a metal foil with alpha particles to observe how they scattered. They expected all the alpha particles to pass straight through with little deflection, because Thomson's model said that the charges in the atom are so diffuse that their electric fields could not affect the alpha particles much. However, Geiger and Marsden spotted alpha particles being deflected by angles greater than 90°, which was supposed to be impossible according to Thomson's model. To explain this, Rutherford proposed that the positive charge of the atom is concentrated in a tiny nucleus at the center of the atom.[13]
Discovery of isotopes
While experimenting with the products of radioactive decay, in 1913 radiochemistFrederick Soddy discovered that there appeared to be more than one type of atom at each position on the periodic table.[14] The term isotope was coined by Margaret Todd as a suitable name for different atoms that belong to the same element. J.J. Thomson created a technique for isotope separationthrough his work on ionized gases, which subsequently led to the discovery of stable isotopes.[15]
Bohr model
Main article: Bohr model
In 1913 the physicist Niels Bohr proposed a model in which the electrons of an atom were assumed to orbit the nucleus but could only do so in a finite set of orbits, and could jump between these orbits only in discrete changes of energy corresponding to absorption or radiation of a photon.[16] This quantization was used to explain why the electrons orbits are stable (given that normally, charges in acceleration, including circular motion, lose kinetic energy which is emitted as electromagnetic radiation, see synchrotron radiation) and why elements absorb and emit electromagnetic radiation in discrete spectra.[17]
Later in the same year Henry Moseleyprovided additional experimental evidence in favor of Niels Bohr's theory. These results refined Ernest Rutherford's and Antonius Van den Broek's model, which proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. Until these experiments, atomic number was not known to be a physical and experimental quantity. That it is equal to the atomic nuclear charge remains the accepted atomic model today.[18]
Chemical bonding explained
Chemical bonds between atoms were now explained, by Gilbert Newton Lewis in 1916, as the interactions between their constituent electrons.[19] As the chemical properties of the elements were known to largely repeat themselves according to the periodic law,[20]in 1919 the American chemist Irving Langmuirsuggested that this could be explained if the electrons in an atom were connected or clustered in some manner. Groups of electrons were thought to occupy a set of electron shells about the nucleus.[21]
Further developments in quantum physics
The Stern–Gerlach experiment of 1922 provided further evidence of the quantum nature of atomic properties. When a beam of silver atoms was passed through a specially shaped magnetic field, the beam was split in a way correlated with the direction of an atom's angular momentum, or spin. As this spin direction is initially random, the beam would be expected to deflect in a random direction. Instead, the beam was split into two directional components, corresponding to the atomic spin being oriented up or down with respect to the magnetic field.[22]
In 1925 Werner Heisenberg published the first consistent mathematical formulation of quantum mechanics (Matrix Mechanics).[18]One year earlier, in 1924, Louis de Broglie had proposed that all particles behave to an extent like waves and, in 1926, Erwin Schrödinger used this idea to develop a mathematical model of the atom (Wave Mechanics) that described the electrons as three-dimensional waveforms rather than point particles.
A consequence of using waveforms to describe particles is that it is mathematically impossible to obtain precise values for both the position and momentum of a particle at a given point in time; this became known as the uncertainty principle, formulated by Werner Heisenberg in 1927.[18] In this concept, for a given accuracy in measuring a position one could only obtain a range of probable values for momentum, and vice versa.[23] This model was able to explain observations of atomic behavior that previous models could not, such as certain structural and spectral patterns of atoms larger than hydrogen. Thus, the planetary model of the atom was discarded in favor of one that described atomic orbitalzones around the nucleus where a given electron is most likely to be observed.[24][25]
Discovery of the neutron
The development of the mass spectrometerallowed the mass of atoms to be measured with increased accuracy. The device uses a magnet to bend the trajectory of a beam of ions, and the amount of deflection is determined by the ratio of an atom's mass to its charge. The chemist Francis William Astonused this instrument to show that isotopes had different masses. The atomic mass of these isotopes varied by integer amounts, called the whole number rule.[26] The explanation for these different isotopes awaited the discovery of the neutron, an uncharged particle with a mass similar to the proton, by the physicist James Chadwick in 1932. Isotopes were then explained as elements with the same number of protons, but different numbers of neutrons within the nucleus.[27]
Fission, high-energy physics and condensed matter
In 1938, the German chemist Otto Hahn, a student of Rutherford, directed neutrons onto uranium atoms expecting to get transuranium elements. Instead, his chemical experiments showed barium as a product.[28][29] A year later, Lise Meitner and her nephew Otto Frischverified that Hahn's result were the first experimental nuclear fission.[30][31] In 1944, Hahn received the Nobel prize in chemistry. Despite Hahn's efforts, the contributions of Meitner and Frisch were not recognized.[32]
In the 1950s, the development of improved particle accelerators and particle detectorsallowed scientists to study the impacts of atoms moving at high energies.[33] Neutrons and protons were found to be hadrons, or composites of smaller particles called quarks. The standard model of particle physics was developed that so far has successfully explained the properties of the nucleus in terms of these sub-atomic particles and the forces that govern their interactions.[34]
Subscribe to:
Posts (Atom)
Decorative painting works
Decorative paint work is one of the modern and current/trading paints work it has many advantages over normal paint work which help in hav...

-
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and ...